 SNOW HYDROLOGY (GEOG 4321): MOUNTAIN SNOWPACK
SNOW HYDROLOGY (GEOG 4321): MOUNTAIN SNOWPACK  SNOW HYDROLOGY (GEOG 4321): MOUNTAIN SNOWPACK
SNOW HYDROLOGY (GEOG 4321): MOUNTAIN SNOWPACK
 Sampling a snowpit, Sierra Nevada, CA.
Sampling a snowpit, Sierra Nevada, CA.
Snow on the ground is a dynamic medium. The properties and characteristics of fallen snow change constantly as a function of energy fluxes, wind, moisture, water vapor, and pressure. For example, the time window for good skiing in mid-winter may last several days after a fresh snowfall in cold continental climates. During spring conditions the time window may be only an hour or two as rock-hard boilerplate turns to ideal corn conditions before additional energy inputs turns the snowpack to unskiable slush. Physical properties of snow change over time. Furthermore, snow properties can vary widely over small distances, both vertically within a snowpack and horizontally over space.
The purpose of this chapter is to provide a qualitative overview of the material properties of snow on the ground. We will also introduce some of the processes that modify snow on the ground. The physical properties of snow on the ground may differ greatly from the ice crystals in the snowfall events. Ice particles that form in the atmosphere have a large variety of crystal shapes and sizes. By the time these ice crystals reach the ground, they have already undergone a number of changes resulting from growth, disintegration, or agglomeration. For example, high wind speeds near the surface may break ice crystals into smaller pieces. Further disintegration of the original snow crystals may occur as the crystals are bounced and dragged over the snow surface in the turbulent boundary layer. After being reduced in size and shaped more symmetrically through interactions with wind, the individual crystals may be packed more closely to produce a much denser surface layer than would otherwise occur.
Once ice particles are deposited in a snowpack, the shape and size of the ice grains can change dramatically by a process called snow metamorphism. Snow metamorphism is analogous to metamorphism in geology, where changes in form occur because of heat flow and pressure. A snowcover is the result of numerous snowfall events. Each of these snowfall events may occur with different meteorological conditions, resulting in a horizontally stratified snowpack that is vertically heterogeneous or anisotropic. Furthermore, meteorological conditions between snowfalls will change the physical character of the snowpack. For example, warm, sunny days may cause melt at the snow surface, producing a high-density, well-bonded layer over a colder, lower-density, and relatively poorly-bonded layer. Furthermore, ice crystals in the atmosphere are formed in a highly supersaturated, thermodynamically unstable environment. Once these ice crystals are deposited on the ground, they will change quickly to minimize their surface free-energy. Snowpack stratigraphy is the result of a combination of processes, which include: (1) individual snowfalls each with different meteorological conditions, (2) meteorological conditions between snowfall events which can form sun and wind crusts, and (3) different rates and types of grain growth after snow accumulates in the snowpack.
Snow on the ground is a complicated mixture of water in three phases: ice, liquid water, and water vapor. Moreover, air is an important component of snow on the ground, comprising 95% or more of the volume of champagne powder that is ideal for skiing. Additionally, solutes and particulates within the snowpack can influence snow properties such as the albedo of snow. Once ice crystals are deposited on the ground, we refer to them as snow grains because of the changes that occur within the snowpack. Snow grains can become bonded to their neighbors, a process called sintering. These bonded snow grains act as an ice skeleton to provide structural strength to the snowpack. As temperature within a snowpack reaches 0ēC, the ice particles will begin to melt.
The best way to understand and measure snowpack properties is to dig a snowpit. A snowpit is constructed by digging a hole from the snow surface to the ground. The size of the hole is determined by (a) the number and (b) type of measurements you will make, as well as (c) the number of power bars you've consumed. Digging snow pits is hard work!
The most important tool in snow hydrology is a shovel. Generally one side of the hole is designated the working wall, where measurements of snow properties will be made. The working wall is on the side of the snowpit facing the sun, so that the wall itself faces away from direct sun and is self-shaded. After the snowpit is constructed, the working wall is smoothed by shaving back the wall with a shovel with a square blade. One drawback is that construction of a snow pit and analysis of a snow profile on the working wall takes on the order of one to two hours, depending on snow depth and hardness.
Once we've constructed our snowpit, the depth of the snowpack is an obvious parameter, easily measured by placing a measuring tape from the snow surface to the ground. When measuring snow depth in a snowpit, we always place the zero mark at the snow ground interface rather than the snow surface. The reason is because the snow-ground interface never changes in height, while the snow surface does change in height relative to the ground. Snow depth can be highly variable over space. In mountainous terrain snow depth can vary as much as 10 cm to 10 meters (two orders of magnitude) over a distance of 100 meters. Estimating snow depth on the Colorado Plateau by poking a ruler through the snowpack every 10 meters is impractical.
We'd also like to know the amount of ice relative to air in the snowpack, or the density of the snow. Density is defined as the amount of mass per unit volume. We generally use the units of kilograms per cubic meter (kg m^3), which is 1,000, the value expressed in grams per cubic centimeter (g cm^3). Density is measured by weighing a known volume of snow. The density of snow typically ranges from 30 kg m^3 for the driest champagne powder to 600 kg m^3 during snow melt runoff. The most important hydrologic parameter is the amount of liquid water stored as snow, the snow water equivalence (SWE). We can calculate SWE for some unit area as the product of snow density (rs) and depth (h), divided by the density of water (rw=1,000 kg m^3).
Alternatively, we might be interested in the porosity of snow on the ground. The ratio of the density of dry snow to the density of ice (ri=917 kg m^3) gives the fraction of the volume of a snow sample that is composed of ice. When this fraction is subtracted from 1, we obtain the fraction of the snow sample that is composed of air, or the porosity of the snowpack.
Champagne powder with a density near 30 kg m^3 is 97% air and only 3% ice! The densest dry snow at about 550 kg m^3 has a porosity of 40%; the ice particles are as close together as randomly packed, randomly shaped objects can be.
Often we are interested in how strong the snowpack is, from an avalanche standpoint or perhaps because we want to walk or ski on the snow surface. Hardness provides a measure of the strength of the snowpack in compression. Hardness has units of force per unit area, N m^2. Hardness generally increases with increasing density in a snowpack, and since density generally increases with depth, because of compression from the overlying snow, hardness generally increases with depth. Hardness can be measured using several types of gauges. In most field situations, hardness is estimated using a series of hand tests that involve pressing objects until the snowpack fails in compression.
The type and size of individual grains within the snowpack influences a variety of processes, including the amount and strength of bonding within the snowpack and the rate and location of meltwater flow through snow. The growth rate and grain type within a dry snowpack is driven primarily by gradients of water vapor within the snowpack. In turn, vapor pressure gradients depend on four important variables: (1) initial type of ice particle and the conditions under which it was deposited in the snowpack, (2) the temperature of the snowpack, (3) the magnitude of any temperature gradient, and (4) the size of pore spaces. Grain size and type are estimated through use of a crystal card with a millimeter grid and an 8-10x hand lens.
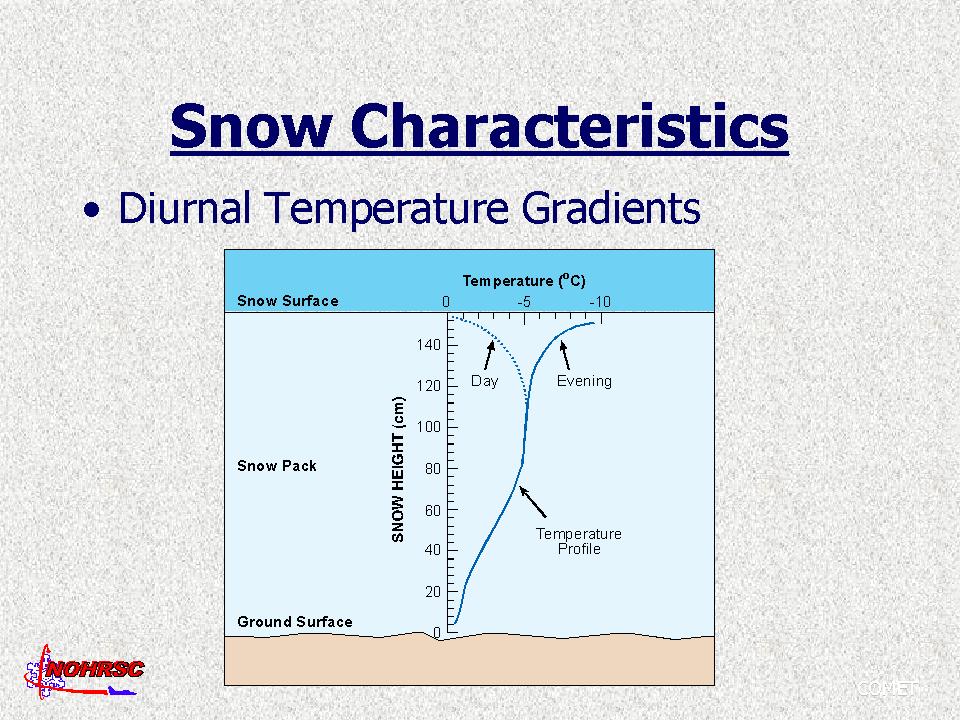
The temperature of the snowpack is an important variable for a variety of reasons. Temperatures within a snowpack are generally not uniform from top to bottom, which leads to gradients in the movement of water vapor through the snowpack. We'd like to know when the snowpack becomes isothermal at 0ēC and meltwater is released from the bottom of the snowpack to the hydrologic system. Snowpacks are bounded by the atmosphere above and in seasonally snow-covered areas by ground below. In general, the basal layer at the bottom of the snowpack is warmed to near 0ēC because of: (1) stored head in the ground from summer warming (most important), and (2) geothermal heat from the interior of the earth. The temperature of the snow surface responds to prevailing synoptic conditions as well as daytime heating and nighttime cooling (diurnal fluctuations). The combination of these boundary conditions usually results in a snow surface that is cooler than the bottom of the snowpack, producing a vertical gradient in temperature. The temperature of the snowpack can be measured using thermometers, thermisters, and thermocouples.
Here's an example from Red Mountain Pass, Colorado.
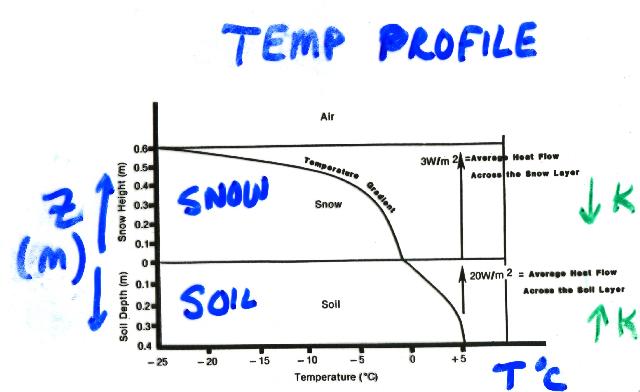 This is from Figure 4 of Armstrong's PhD thesis.
These are temperatures and associated heat flow conditions
(averaged across the individual layers) typical of a high-altitude
continental site in early winter during the coldest portion of the diurnal
cycle.
The average heat flow across the soil layer is towards the snowpack
at about 20 W/m2.
Because of the insulating properties of the snow,
the average heat flow through the snowpack is only 3 W/m2.
This is from Figure 4 of Armstrong's PhD thesis.
These are temperatures and associated heat flow conditions
(averaged across the individual layers) typical of a high-altitude
continental site in early winter during the coldest portion of the diurnal
cycle.
The average heat flow across the soil layer is towards the snowpack
at about 20 W/m2.
Because of the insulating properties of the snow,
the average heat flow through the snowpack is only 3 W/m2.
The bottom line is that the temperature of the snowpack changes with depth. And perhaps most importantly and counter-intuitively, the snowpack is generally warmest at the BOTTOM of the snowpack, not the top!
The amount of water vapor in the snowpack is an important property that is difficult to measure. There is considerable mass exchange across pore spaces within a snowpack by sublimation, vapor diffusion, and redeposition. As a rule of thumb, some snow researchers believe that every snow grain in the snowpack sublimates, diffuses in the gas phase to somewhere else in the snowpack, and is then redeposited. The relative humidity within the snowpack is always very close to 100% because pore spaces in snow are poorly ventilated. Since the pore space within the snowpack is at or very near the saturation vapor pressure, the pressure of water vapor within the snowpack is controlled by the temperature of the snowpack. Warmer areas within the snowpack will have higher vapor pressure than colder areas of the snowpack, because both areas are at the saturation vapor pressure for their respective temperatures. Water vapor then diffuses from warmer (higher vapor pressure) to colder (lower vapor pressure) areas of the snowpack. Since the relative humidity of the snowpack is always about 100%, once we measure snow temperature we can estimate vapor pressure using the Clausius-Clapeyron equation.
Here we provide a brief overview of energy exchange at the snow surface and in the snowpack. The presence of snow on the ground produces large changes in the energy transfers between the surface and the atmosphere. Snow on the ground generally absorbs and transmits less solar radiation, reduces air movement, and conducts less heat. Once energy is gained or lost at the snow surface, the energy is transferred within the snowpack primarily by two mechanisms: (1) conduction through the ice skeleton, and (2) vapor diffusion through the pore spaces. Energy exchanges at the snow-atmosphere interface are driven primarily by radiation and turbulent fluxes. The turbulent fluxes, sensible and latent energy exchanges, may be large in magnitude but are generally opposite in sign. Consequently, they tend to cancel each other out. Therefore, we will concentrate on radiation fluxes at the snow-air interface.
All objects at all times at all temperatures radiate (emit) electromagnetic energy as well as absorb electromagnetic energy from their surroundings. Radiative energy transfers occur continuously, even after the sun has dipped below the horizon and daylight switches to night-time. The amount of radiation emitted by an object is a function of the temperature of the object. Short-wave or solar radiation is emitted by the sun in the wave band from about 0.4 to 5.0 um. Emission of short-wave radiation from the snowpack is negligible. Long-wave radiation is emitted by the atmosphere and the earth from approximately 3 to 100 um. During the day, we see the consequences of sunlight striking the snowpack and changing champagne powder to Sierra cement. However, there is always unseen radiation as well, long-wave radiation. Long-wave radiation is unseen and not as intuitive as solar radiation, but as we will see the loss of energy from the snow surface by long-wave radiation has important effects on snow temperature and grain type.
A large portion of the short-wave radiation that strikes a snow surface is reflected back to the atmosphere. The ratio of the reflected to incoming radiation is called the albedo, and is expressed as a fraction or percentage. The albedo of snow can be as large as 95% for new, dry snow, one of the highest values for any substance on the surface of the earth. The solar radiation that is not reflected can penetrate the snowpack, with the intensity of radiation decreasing exponentially with depth. For dry snow with a density of about 100 kg m^3, less than 10% of solar radiation that penetrates the snowpack remains after a distance of 10 cm. Solar radiation during the day is thus a source of energy that can raise the temperature of the upper layers of the snowpack.
The snow surface is almost a perfect black-box with respect to adsorption and emittance of long-wave radiation. Incoming long-wave radiation is absorbed at the snow surface with negligible penetration into the snowpack. Long-wave radiation is lost continuously from the snowpack at the snow surface. Incoming long-wave radiation comes from atmospheric sources, primarily water vapor and to a lesser extent carbon dioxide. The snow surface also continuously looses energy by emitting long-wave energy, which cools the snow surface.
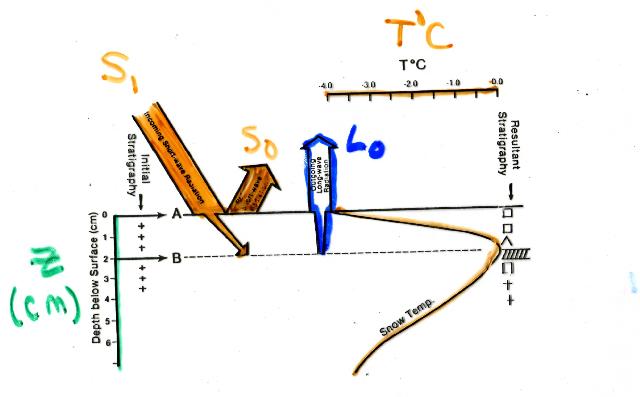
Here's a schematic summary of the fluxes involved in surface radiation and heat exchange at an "ideal" snow-covered site, based on figure 35 of Armstrong's PhD dissertation. Q star is the net energy flux. Sensible (QH) and latent heat (QE) has similar magnitudes but opposite signs during the day, so they tend to cancel each other out. At night, they have the same sign towards the snowpack, which means condensation is occuring. The active surface is the location of maximum energy change within the system. During the day it is not the snow surface, but several centimeters below the snow surface because of penetration to those depths by incoming short-wave radiation. Energy is lost to the atmosphere from the surface of the snowpack by long-wave radiation. During the day, this loss may cancel any energy increase from incoming solar radiation. At night, there is no solar radiation input, but there is still a loss of energy from long-wave radiation. At night, the net energy balance of the snow surface is generally negative, and the surface temperature will actually be lower than air temperature.
The rate of grain growth and type is to a large extent driven by net radiative fluxes. On a clear, cold day in mid-winter, net radiation on south-facing slopes is generally positive and the top of the snowpack can become warmer. On the same day, the snow surface can become colder on steep north-facing slopes because of a lower adsorption of solar radiation combined with higher losses of energy from the emission of long-wave radiation. During a clear night, almost all long-wave radiation emitted from the snow cover is lost to space, with negligible inputs of solar radiation. The snow surface may then be 5 ēC to 20ēC colder than the overlying atmosphere. On a diurnal basis, temperatures near the snow surface in mid-latitude areas may range from -15ēC to 0ēC.
Snow on the ground is difficult to classify because it is constantly changed by variations in temperature, wind, moisture, and vapor pressure. Snow riders have developed a lexicon for snow that provides descriptive information: champagne powder, buffed snow, cold smoke, corduroy, crud, mashed potatoes, Sierra cement, breakable crust, boilerplate. Eskimos supposedly have over a hundred words for snow, such as aput (snow on the ground), qana (falling snow), piqsirpoq (drifting snow), and qimuqsuq (snow drift), none with a common root. Providing a rigorous scientific definition of snow on the ground has proven to be difficult. There is still debate about what to call the individual components of the snowpack. Snow on the ground is a polycrystalline material composed of ice crystals, metamorphosed snow, and ice. Furthermore, once sintering begins in the snowpack, individual components are difficult to detect under field conditions. Sommerfeld and LaChapelle recommend using the term grain to identify the visible ice particles in a snowpack, and grain boundary to designate where two or more grains are sintered together. We've adapted that approach here.
One of the most important characteristics of the snowpack are the size and shape of individual snow grains.
Grain shape and size tells us about the history of the snowpack at that site. Grain shape and size provide important information about snow stability and potential for avalanches to occur. There are different ideas on how to classify snow morphology. Part of the issue is the same as in biology: are you a splitter or a lumper? Perhaps the best guide is "The international classification for seasonal snow on the ground" by Colbeck and others. This report can by found at the National Snow and Ice Data Center in Boulder, CO.
There are general characteristics of snow grains that we can evaluate. What is the basic shape of the snow grains within a layer? Are they solid, hollow? Are they rounded? Do they have sharp edges? Are they faceted? Have they been broken by wind, abraded, rimed? A 10x hand lens is all you need to look at the morphology of snow grains.
Here are some examples of the most common types of snow grains.
New improvements in scanning electron microscopy (SEM) have provided the ability to make the photos above. These photos are from a trip to Niwot Ridge to collect snow samples for SEM analysis by the SEM team headed by Bill Wergin.
How big are snow grains? Most ET grains are less than 0.5 mm in diamter. In contrast, TG grains are generally larger than 3.0 mm in diameter.
Rounded snow grains that approximate the shape of spheres are characteristic of snowpacks with temperature gradients less than 10ēC m^-1. Snow metamorphism is driven by gradients in vapor pressure, which in turn are driven by temperature gradients. Small temperature gradients result in small vapor pressure gradients and slow grain growth within the snowpack. The result is the formation of rounded snow grains that tend to be 0.1 to 0.2 mm in diameter. These rounded grains tend to bond together into simple chains that provide structural stability to the snowpack. The process of forming these snow grains has a number of synonyms, including the historical term of equitemperature metamorphism (ET metamorphism), equilibrium metamorphism, radius-dependent metamorphism, radius of curvature metamorphism, and destructive metamorphism. One explanation for the formation of rounded snow grains is that vapor diffusion within the snowpack causes a loss of mass from points on individual snow grains to gains in mass in hollows.
Depth hoar forms in areas of a snowpack where temperature gradients are greater than 10ēC m^-1. The process of forming these snow grains has a number of synonyms, including the historical term of temperature gradient metamorphism (TG metamorphism), constructive metamorphism, and kinetic growth. The large temperature gradient induces a large gradient in vapor pressure, such that water vapor moves from warmer areas of the snowpack with relatively higher vapor pressures across pore spaces to colder areas of the snowpack with lower vapor pressure. These conditions produce angular or faceted grains, which may later develop steps and striations on their surface, resulting in cup-shaped crystals with a hollow center that generally range in size from 3-8 mm. Under very favorable conditions, individual grains can be larger than 15 mm. These large, angular grains result in poor sintering, low cohesion, and little structural strength, earning an additional synonym of sugar snow. It is almost impossible to make a snowball out of depth hoar because of the low amount of sintering. Depth hoar is often the weakest layer in the snowpack and is a potential sliding layer for avalanches.
I've chosen to emphasize some of the types of snow that can be found at or near the snow surface because of the many unusual shapes here caused by the very dynamic energy exchanges.
Overview and summary table(not comprehensive).
Large, feather-shaped crystals termed surface hoar may grow at the snow-air interface during clear and cold nights. Surface hoar is essentially frozen dew formed by vapor deposition onto a radiation-cooled surface. The crystals have a complicated, dendritic shape that resembles feathers growing from the surface of the snowpack into the surrounding atmosphere, as in the example below.

The length of these crystals usually ranges from 1 to 10 mm, but can be much longer under ideal conditions for formation. Surface hoar generally forms a layer at the snowpack surface several millimeters to centimeters in thickness. Density is generally quite low, 100 to 200 kg m^3. Skiing on surface hoar can be quite nice. When buried by subsequent snowfalls, surface hoar is a potential weak layer in the snowpack. Buried surface hoar is extremely efficient in propagating shear instabilities. Fatal avalanches have resulted when skiers on flat terrain caused shear fractures in buried surface hoar, which propagated upslope to undercut a slab. Because the layer of surface hoar becomes quite thin when compressed by overlying snow, this potential sliding layer is quite difficult to recognize when buried.
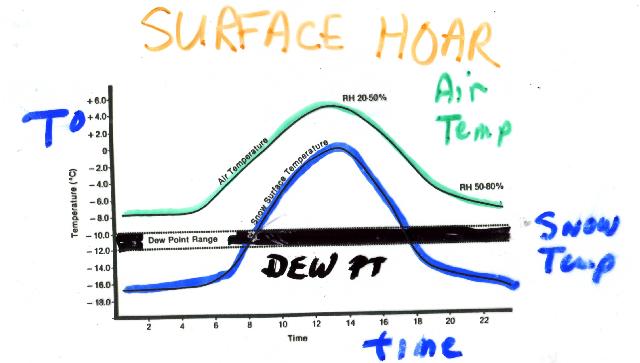
Adapted from Figure 32 of Richard Armstrong's PhD thesis.
Surface hoar forms when heat loss by long-wave radiation at the snow surface lowers the surface temperature below the air temperature. Deposition then occurs onto the snow surface as relative humidity exceeds 100% with air temperature below 0ēC. Surface hoar generally occurs during clear, cold nights, often when there is an inversion layer in the overlying atmosphere. A warm and moist air mass during the day facilitates the formation of surface hoar by raising the air temperature and increasing the specific humidity of the atmosphere. Little or no wind is important, otherwise the temperature loss caused by longwave radiation from the snow surface will be offset by energy transfers from latent and sensible heat.
Examples
Summary:
are hard, well-bonded layers formed at or near the snow surface. Density ranges from about 200 kg m^3 to as high as 800 kg m^3. Hardness in turn varies widely, from finger hardness to armor-plated. Meltwater in spring snow that refreezes during a cold night provides a snow surface that is hard enough for cars to drive on. Don't try it. We^ve rescued a few fisherman who^ve driven several miles on the snow surface early in the morning to their favorite fishing spots, only to see their cars high-centered on their oil pans as they come back for the second six-pack of their favorite beverage. Skiing on surface crust can range from outstanding to the worst. When a hard meltwater crust is softened in the mid-morning by incoming solar radiation, the resulting spring skiing can make a novice skier look like an Olympic champion. Alternatively, when a melted surface layer only partially refreezes, the resulting breakable crust may be sufficiently strong to support a skier until a ski is weighted in a turn, at which time the lead ski breaks through the crust into the underlying soft snow, sinks, and pitches the skier head-first.
Surface crusts that become buried by subsequent snowfalls are a major cause of snow stratigraphy. Causes for the formation of surface crusts include: (1) surface melting and refreezing, (2) rain on snow events, (3) wind deposition, and (4) wind erosion of the snow surface. The buried crust may act as a sliding layer for avalanches, since the buried layer may be stronger than the surrounding snow. Furthermore, bonding on either side of the crust to the adjacent snow may be poor because the layer is so smooth, further increasing the risk of avalanche danger. Buried crusts may also play an important role in meltwater flow through snow. As liquid water percolates downward through the snowpack and encounters a buried crust, it may pool and move laterally until breakthrough is achieved in a vertical flow channel.
 Firnspiegel with shadow of hiker.
Image from FIRNSPIEGEL LLC.
Firnspiegel with shadow of hiker.
Image from FIRNSPIEGEL LLC.
Conditions for firnspeigel generally occur during the snow melt season. At night liquid water freezes on the snow surface as the loss of long-wave radiation cools the snowpack surface below freezing. The thin ice acts like a greenhouse the next day as solar radiation increases in the morning. Short-wave radiation penetrates the thin ice, melting the snow underneath but not the ice cover. The liquid water produced by heating from solar radiation infiltrates into the underlying snow. The mass loss causes an air gap to form between the ice layer and the snowpack. If you look closely at firnspeigel during the day, you can see liquid water droplets on the underside of the ice layer. The presence of the liquid droplets is because water vapor rising from the snowpack contacts the impenetrable ice layer and condenses.
Examples
Radiation recrystallization is caused by solar radiation penetrating the snow surface and warming the snowpack below. The surface of the snowpack is cooled through the loss of energy from emission of long-wave radiation. The cooling of the snow surface and warming of the snowpack several cm below the snow surface results in a large temperature gradient. The resulting gradient in vapor pressure produces faceted crystals. Conditions for radiation recrystallization are similar to diurnal temperature gradients, and are facilitated by low atmospheric humidity and occur best at high elevation because of the intense solar radiation.
Below is an example of radiation and heat exchange conditions which would lead to the formation of near-surface faceted crystals (Figure 37, Armstrong's PhD dissertation).

This example is typical of those measured at mid-day during springtime under clear skies at 3,400 m in the San Juan Mountains, Colorado. Note that the maximum snow temperature is near 0 degrees C at the active layer 2 cm below the surface of the snow.
There are three subclasses of near-surface faceted crystals. Below is summary information on those three types.
Summary: Radiation Recrystallization
Summary: Diurnal Recrystallization
Summary: Melt-Layer Recrystallization
Sastrugi are ridges of snow formed when wind erodes and drifts the snow. Sastrugi is generaly a series of long, frequently sharp, wave-like ridges of hard snow characteristic of windswept alpine and polar plains when the winds blow continually from one direction. Sastrugi are oriented perpendicular to the wind with a gentle slope to windward and a steep slope to leeward.
Examples