 SNOW HYDROLOGY (GEOG 4321): ICE PHYSICS
SNOW HYDROLOGY (GEOG 4321): ICE PHYSICS  SNOW HYDROLOGY (GEOG 4321): ICE PHYSICS
SNOW HYDROLOGY (GEOG 4321): ICE PHYSICS
Name | Abbrev | Electrons | Protons | Neutrons |
|---|---|---|---|---|
| Hydrogen | H | 1 | 1 | 0 |
| Deuterium | D | 1 | 1 | 1 |
| Tritium | T | 1 | 1 | 2 |
| Oxygen-16 | 16O | 8 | 8 | 8 |
| Oxygen-18 | 18O | 8 | 8 | 10 |
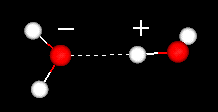
The hydrogen bond can be regarded as a weak covalent bond, meaning that the energy needed to break it is low - up to 40 KJ/mol, compared with 492 KJ/mol to break one H-O bond in H-O-H. This is the reason why hydrogen bonds ``flicker'' on and off rapidly in liquid water.

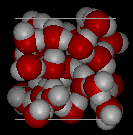
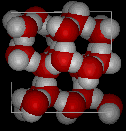
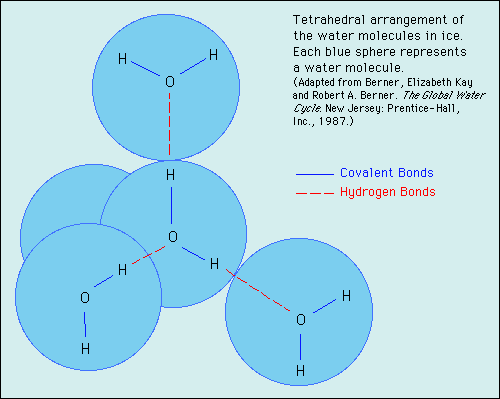
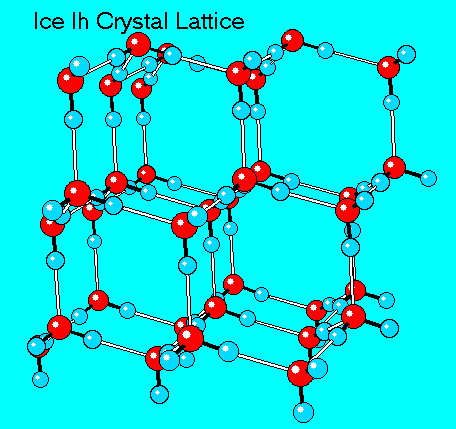
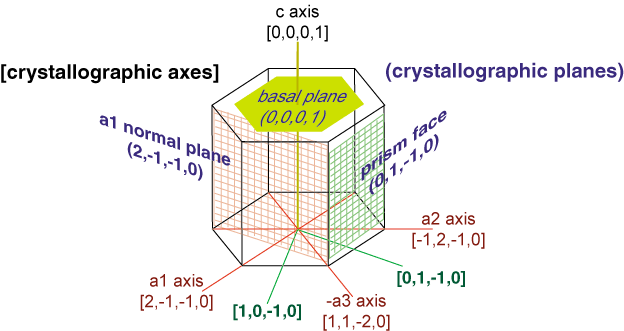
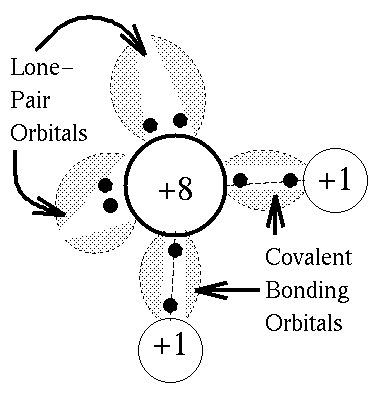
The solid to liquid phase transition is the melting process of ice. Any phase transition occurs due to a change in kinetic energy of the participating particles. If the system is in the solid phase and the kinetic energy is sufficiently increased, the system will go from solid to liquid. In the solid phase, the network strives for the geometrically and bondwise most favoured setup, meaning the lowest energy conformation. In ice, a water molecule has four nearest neighbors to which it is bonded via hydrogen bonds (two from its hydrogen atoms and two from the lone electron pairs on the oxygen). The geometry leads to a rather open hexagonal structure, each of the four bonds representing a lowered overall energy. When the average kinetic energy is raised, the additional jostling begins to destroy the open hexagonal structure. Paradoxically, this allows the molecules to move closer to each other, making and breaking hydrogen bonds much more rapidly. On average, there can now be more than four nearest neighbors at a time, lower energy, and a higher density in the just-melted liquid system. Consequently, in liquid water each molecule is hydrogen bonded to approximately 3.4 other water molecules. In ice each each molecule is hydrogen bonded to 4 other molecules. Therefore the density of ice Ih is 917 kg/m3, compared with a density of 1,000 kg/m3 for liquid water at 4 degC. When rising temperature increases average kinetic energy yet further, molecules move through the liquid so fast that fewer bonds are formed at any one time, shortening the average time each bond exists. The result is higher total energy and thus lower density. So at atmospheric pressure the temperature of greatest density is just above the melting point, at 4 degC Celsius.
Coined by Faraday in the 19th century, quasi-liquid is a term describing
liquid-like layers that form on solid surfaces at temperatures below the
solid's melting point.
The quasi-liquid-like layer of the surface of ice at temperatures
near the melting point may be a key to many of the
processes in snow hydrology.
(This section adapted in part from
http://electron.lbl.gov/graphs/figures/icetemp.html
and http://pearl1.lanl.gov/external/Research/chem_dynamics.htm)
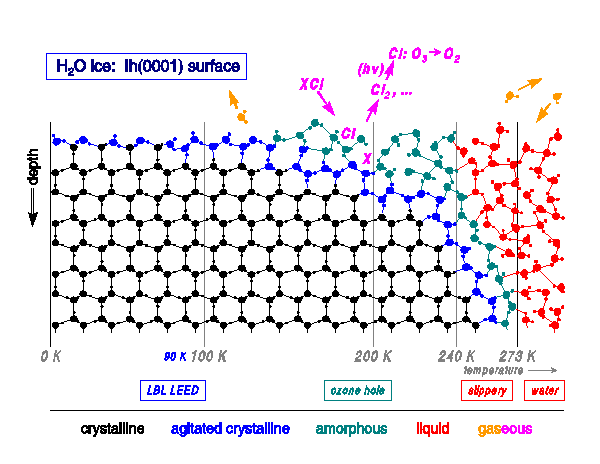
Our proposed evolution of the surface structure of ice is shown
with real data from Antarctica as
temperature rises from absolute zero (at left) to above the bulk
melting point (273 K, at right).
Large vibrational
amplitudes of the outermost water molecules, still in the crystalline state
(blue),
exist at extremely cold temperatures.
As temperature rises, molecular agitation induces more crystalline
defects, yielding an amorphous or quasi-liquid layer (green),
as well as evaporation (orange).
One important consequence is that,
at about 190 K, ice in the Antarctic polar stratospheric clouds
catalyzes Cl-containing molecules to Cl2,
which in turn is dissociated by sunlight into Cl atoms
that decompose ozone
(O3):
the catalytic action may be due to the amorphous or quasi-liquid
ice surface.
Above about 240 K (-30 ºC, -20 ºF), the ice surface probably is liquid (red),
to a thickness of 10s to 100s of Å:
this permanent liquid film can
explain the slipperiness of ice.
The quasi-liquid like layer may also explain many of the properties
of snow in the atmosphere as well,
such as how snowflakes may get larger due to mechanical locking.
For those interested, here's a cool site that chats about two potential unique applications of the quasi-liquid like layer:
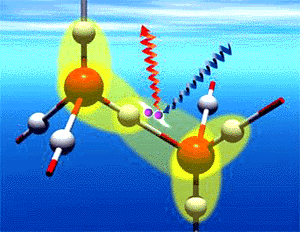
Supplemental information. As mentioned above, the quasi-liquid layers that form on atmospheric ice particles and in the world's snow pack affect the chemistry of those systems and have far-reaching environmental implications. Thus, from being a mere scientific curiosity, the study of these layers has become an inquiry into the mechanisms that drive global change. Preliminary results suggest that both the uptake of organic compounds in snow and the chemistry of tropospheric ozone are affected by the presence of quasi-liquid layers. By studying quasi-liquids in the laboratory with optical techniques, Los Alamos chemists have been able to develop a thermo-dynamic model that predicts the total quasi-liquid volume in the atmosphere and in the snow pack.

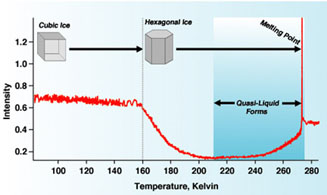
Left: The Los Alamos model suggests that quasi-liquids form in multilayered islands on ice surfaces below the triple point, where solid, liquid, and vapor are in equilibrium.
Right: Ice crystals change from cubic form to hexagonal near 160 K. SHG data supports the Los Alamos model by showing signal for quasi-liquid layers starting at about 212 K and increasing through the melting point.
Snow Crystals Kenneth Libbrechts' site at CalTech. Probably the single best source on ice physics and snow crystals on the web. Much of the material from this unit from his site.
Water Molecule Home Page from NYU/ACF Scientific Visualization Laboratory