 SNOW HYDROLOGY (GEOG 4321): ENERGY BALANCE
SNOW HYDROLOGY (GEOG 4321): ENERGY BALANCE  SNOW HYDROLOGY (GEOG 4321): ENERGY BALANCE
SNOW HYDROLOGY (GEOG 4321): ENERGY BALANCE
Nice overview by Don Cline, required reading .
The optical properties of any medium are expressed by the refractive index:
Snow is a matrix of ice grains and air, and, when at 0deg C, it also has a significant fraction of liquid water. Snow also often contains absorbing impurities such as soot, dust, and pollen. The optical properties thus depend on the bulk optical properties and geometry of the ice grains, the liquid water inclusions, and the solid and soluble impurities. In the visible and near-infrared wavelengths, the bulk optical properties of ice and water are very similar, so the reflectance and transmittance of the snowpack in this region of the electromagnetic radiation spectrum depend on the wavelength variation of the refractive index of ice, the grain size distribution of the snow, the depth and density of the snowpack, and the size and amount of those impurities whose refractive indices are substantially different from those of ice and water.
The most important optical property of ice, which causes spectral variation in the reflectance of snow in visible and near-infrared wavelengths, is that the absorption coefficient (i.e. the imaginary part of the refractive index) varies by seven orders of magnitude at wavelengths from 0.4-2.5 micrometers. Normally, the index of refraction is expressed as a complex number, n+ik. The figures below show the real and imaginary parts of the refractive index for ice and water.
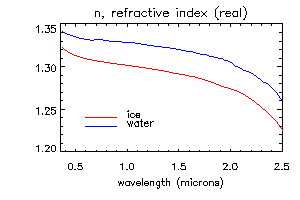
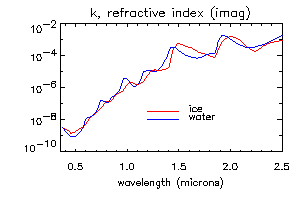
The important properties to note are (1) the spectral variation in the real part n is small and the difference between ice and water may not be significant; (2) the absorption coefficient k of ice and water are very similar, except for the region between 1.35-1.75 micrometers, where ice is slightly more absorptive (3) in the visible wavelengths, k is very small and ice is transparent; and (4) in the near-infrared wavelengths, ice is moderately absorptive, and the absorption increases with wavelength.
Spectral Characteristics of the Reflectance of Snow
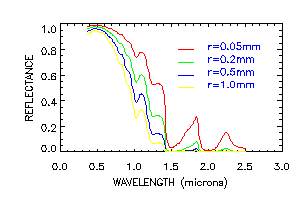
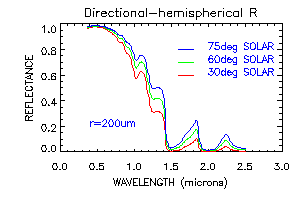
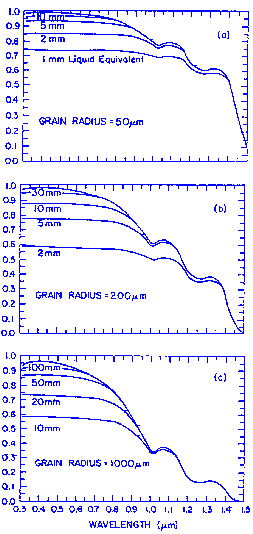
The figure above shows the spectral reflectance of pure deep snow for visible and near-infrared wavelengths, for snow grain radii from 50-1000 micrometers, representing a range for new snow to spring snow, although depth hoar and grain clusters in coarse spring snow can exceed 0.5cm in radius. Because ice is so transparent in the visible wavelengths, increasing the grain size does not appreciably affect the reflectance. The probability that a photon will be absorbed once it enters an ice grain is small, and that probability is not increased very much if the ice grain is larger. In the near-infrared, however, ice is moderately absorptive. Therefore, the reflectance is sensitive to grain size, and the sensitivity is greatest at 1.0-1.3 micrometers Because the ice grains are strongly forward-scattering in the near-infrared, reflectance increases with illumination angle, especially for larger grains (figure below).
The presence of liquid water in the snow does not by itself greatly affect the reflectance. Except for meltwater ponds in depressions, where melting snow overlies an impermeable substrate, liquid water content in snow rarely exceeds 5 or 6%. This small amount of water does not appreciably affect the bulk radiative-transfer properties, except possibly in those wavelength regions where the absorption coefficients of liquid water and ice are appreciably different. Instead, the changes in reflectance that occur in melting snow in large part result from the increased crystal sizes and from an effective size incrase caused by the two-to-four grain clusters that form in wet unsaturated snow. These apparently behave optically as single grains, causing decreased reflectance in near-infrared wavelengths. It has been observed that the spectral reflectance of a snow sample is lower after warm air is blown over it, but that the reflectance does not increase when the snow is refrozen.
There is no explicit dependence on density for the semiinfinite snowpack, up to densities of ~650kg/m3. The natural increases in density are usually accompanied by increases in grain size; hence, there will be a statistical correlation in field observations carried out over a season, but in an experiment where other variables were held constant, spectrally integrated albedo was measured before and after compacting the snow with a snowmobile and no change was found.
In the visible wavelengths, reflectance is insensitive to grain size, but is affected by two variables, finite depth and the presence of absorbing impurities. The transmission of visible light through snow increases with grain size. The figure below shows the relationship of snow reflectance to snow-water equivalence, for a black substrate, i.e., R = 0. For grain radius r = 1.0mm, the reflectance is perceptibly reduced when the snow amount is reduced to 100mm snow-water equivalence.
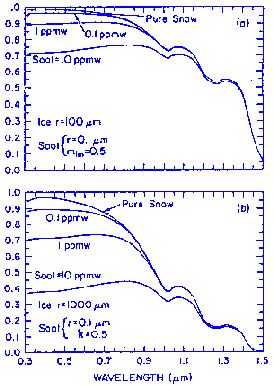
Minute amounts of absorbing impurities have been shown to reduce snow reflectance in the visible wavelengths, where ice is highly transparent. The figure below shows that soot concentrations as low as 0.1ppmw (parts per million by weight) are enough to reduce reflectance. The effect of the absorbing impurities is apparently enhanced when they are inside the snow grains because refraction focuses the light on the absorbers.
Surface interactions
In the interaction of microwaves with snow cover, the basic
electromagnetic properties are the relative dielectric
constants of ice and liquid water and
their geometrical distribution in the snow cover.
The dielectric constant of snow is a weighted average
of the dielectric constants of its components:
air, ice, and liquid water.
This effective dielectric constant determines the
propagation and absorption of microwaves in snow.
By using a dielectric constant that is relative
to that in a real vacuum (the dielectric constant
in air is not significantly different from that
in a vacuum), we can assume a simple relationship between
the complex refractive index of light (m) and
the complex dielectric constant, namely:
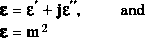
The dielectric constant of snow (epsilon) is equal to the refractive index of light squared, and also has a real and an imaginary component. Measurements of the dielectric component in snow (and soils) are used to measure liquid water content in snow and soil moisture:
Material | Dielectric constant | Reference |
|---|---|---|
| Air | 1.0 | Servay (1990) |
| Ice | 3.2 | Paren (unpublished) |
| Water | 80 | Hasted (1961) |
| Quartz | 4.3 | Gregg (1980) |
Turbulent transfer of energy to and from the snowpack consists of three rates:
Sensible heat is a function of two parameters:
Latent heat is also a function of two parameters:
In short,
So, to understand turbulent fluxes,
we need to understand how the atmosphere is mixed.
Lets start with a simple molecular case,
where all air flow is laminar (eg no mixing).
Heat and water vapor exchange in the laminar
situation is a function of their molecular diffusivities,
which we can write as:
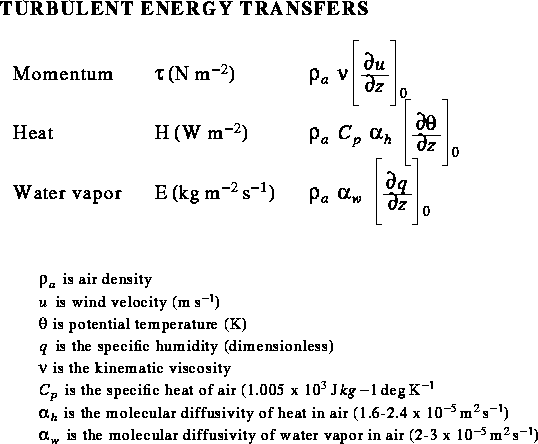
The subscript zero indicates that the gradients are evaluated
at the surface, the snow surface in our case.
Note that all three fluxes are a function of
the density of air,
the rate of movement through the air,
and a gradient with respect to height.
However, these equations are of little practical use,
because of the difficulty in measuring wind speed, temperature,
and humidity gradients in the very thin layer near the
surface where the air flow is laminar.
Let us define a surface layer as the region where the transfers of momentum, heat and water vapor are constant with height. Experimental evidence shows that this layer is perhaps 10-20 m in thickness; operationally we usually define it as the first ten meters above the snow surface. This leads to the simularity hypothesis, whereby we may measure the transfer rates anywhere within this layer and assume that the transfer rates we measure are the same as at the surface.
There are three approaches to measuring the transfer rates within the surface layer:
Measure all three turbulent transfer
components simulaneously.
Measurements are made at one height.
The "best" measurement of turbulent fluxes.
Necessitates specialized instruments with very fast
response times (tenths to hundreths of seconds).
Instrumentation is expensive and fragile.
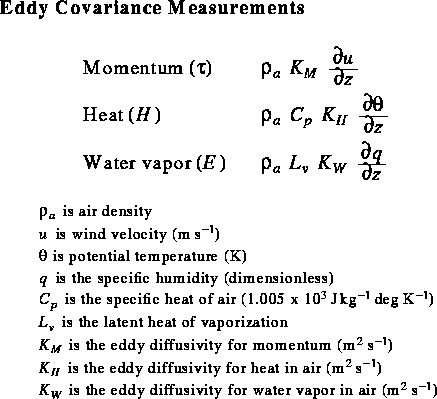
The K terms are effective diffusivity parameters
that incorporate the effect of convection.
Note that they cannot be constant with height,
unless the gradients are constant with height (they generally aren't),
and they may vary by 3 orders of magnitude with height
and with time.
The idea here is to take turbulent flux measurements simultaneously at two or more heights, using standard meteorlogical equipment. Measurements are wind speed, relative humidity, and air temperature. By calculating the difference in these parameters as a function of height above the snow surface, and using the simularity hypothesis, we can then calculate sensible and latent heat fluxes. Generally, you want the measurements to taken fairly close to the ground, where the wind speed difference with height is the greatest. Often measurements are taken at 1 and 2 meters above the snow surface. An obvious problem is that the snow surface height changes continuously with respect to a fixed height. For example, instruments located 1 meter above the snow surface can easily be buried by a large snowfall event.
Mixing rate or momentum transfer between the two measurement heights is a function of shear velocity (u*), hence the K terms from the eddy correlation equations are a function of the shear velocity and the height, or
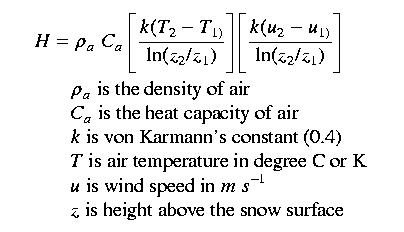
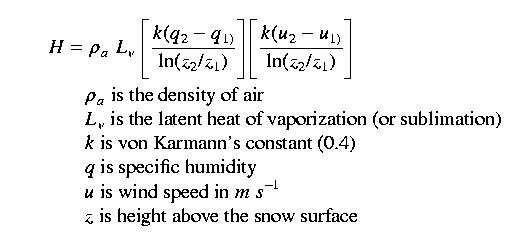
Note that we take the natural log of the measurement
height difference
because of the logarithmic relationship of wind speed with
height.
Keeping two sets of measurement instruments
operating continuously over snow-covered terrain
is a doable but expensive and labor-intensive
enterprise.
Often,
researchers measure temperature and specific humidty
at the snow surface and some fixed height above
the snow surface.
During snowmelt,
we know the snow surface temperature is 0 deg C,
relative humidity is 100%,
and wind speed is zero.
Therefore,
researchers need only measure those parameters at
a single height to use the bulk transfer method:
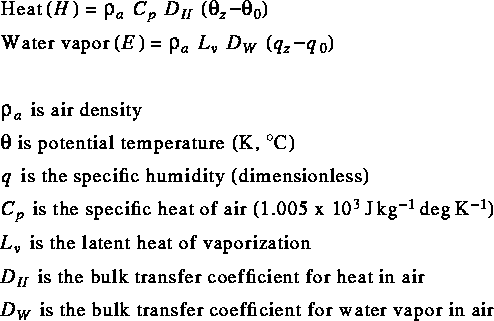
DH and DW are the rate
at which heat and water vapor are moved in
the atmosphere above the snowpack.
The momentum term (tau) is implicitly included
in the bulk transfer coefficients.
An important component of the bulk transfer rates
is the "roughness length" (z0), where
wind speed goes to zero.
The roughness length (in meters) is the height above
the snow surface where the wind speed goes to zero.
As roughness length increases,
so do the turbulent transfer rates.
The assumptions underlying the bulk transfer method lead to a mathematical simplification of turbulent fluxes called the "Bowen Ratio"; the greek letter beta is the symbol for the Bowen Ratio. The Bowen Ratio is the ratio of sensible to latent heat flux, or H/LE. We can write the full equation as:
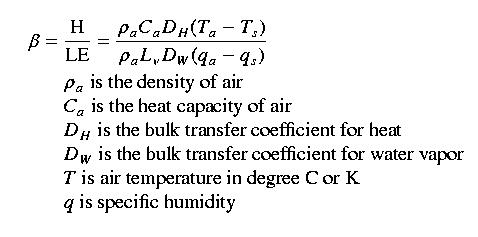
We assume that
DH and DW are the same and hence cancel out.
The density of air also cancels, simplying the ratio to:
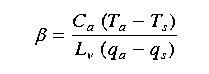
Thus, we can calculate the Bowen Ratio by measuring the air temperature at two heights
and humidity at two heights, since specific heat and latent heat of vaporization are constants.
Moreover, if we assume the snow surface has a temp of 0 degreees C and
a relative humidity of 100% (reasonable assumptions during snowmelt),
then we can calculate the Bowen Ratio simply by measuring air temperature and relative
humidty at one height above the snowpack.
This information is often available from most climate stations.
Another reason for using the Bowen Ratio is that it is dimensionless:
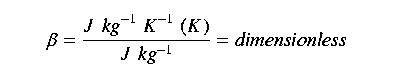
Here's an example of how we can use the Bowen Ratio. Let's assume we've measured net radiaton (R), ground heat flux (G), air temperature and relative humidity. The energy balance equation can be written as:
R + G + H + LE = 0
Now, from the Bowen Ratio (beta = H/LE), we can rewrite and solve for H, or H = LE(beta). Now, we substitute back into our energy balance equation:
R + G + LE(beta) + LE = 0
Now, a little algebra, and we get:
R + G + LE(beta + 1) = 0, and
LE(beta + 1) = -R - G, and
LE = -(R + G)/(beta + 1).
We can now solve for LE using measured R, measured G, and using air temperature and relative humidity to calculate the Bowen Ratio. Once we know LE, we can also solve for H using the relationship H = LE(beta).
Advected energy is another potential source of energy to the snowpack. In general, advected energy is the result of rain-on-snow events. However, there are other ways that energy can be added to the snow, hence the more inclusive term "advected" energy. Advected energy is generally episodic in nature. Generally it can be ignored. However, at times it can be important. For example, most of California's floods are the result of rain-on-snow events. There are two cases:

Using standard values for the parameters (density equals 1000 kg/m3, constants, T = 0 degree C),
for a melting snowpack at 0 degrees C,
where the rain does not freeze, this equation can be reduced to:

For operational purposes, it is usually assumed that Tr is equal
to the air temperature.
The energy added is usually very small. For example, if Tr = 2 degrees C, and P = 2 mm, then A = 16.8 kg/m2day, or 0.19 W/m2. Very small when compared to incident solar radiation over 600 W/m2!.
However, this value can be large if there is lots of warm rain. What happens at times in California is that a cold storm will drop snow to very low elevations, often below 1,000 feet. A warm rain storm than comes in, with T rain 10 or 20 degrees greater than 0 degrees C, up to very high elevations. The A term then does become important, snow melts, and runoff consists of both the new rain and the water stored in the snowpack.
When rain falls on a snowpack which has a temperature below zero degrees C, the situation becomes more complicated. The rain freezes in the snowpack, releasing the latent heat of fusion. This energy can be relatively large. The latent heat of fusion of 335 kJ/kg is much greater than the specific heat of ice at about 1.9 kJ/kg-degree. And remember from an earlier lecture, the energy change is mass times latent heat. For example, 10 mm of rain at zero degrees C added to a 1-m deep snowpack having a density of 340 kg/m3 would, on refreezing, raise the average temperature of the snowpack from -5 to zero degrees C.
Often in operational snow hydrology water managers want to know how much energy is needed before melt starts. The reason is so that they have an idea of when the water stored in the seasonal snowpack will be released to the hydrologic system. Essentially, the snowpack is considered to be an energy reservoir. Once the energy reservoir is full (snowpack isothermal at 0 degrees C), then snow can melt and contribute to the hydrologic system.
The cold content of a snowpack is the amount of energy required to raise its average temperature to the melting point, so

Units are [E L^-2], or using SI units,
J/m2.
Note that the sign is negative: you are taking energy away from the snow
surface to heat the snow below and satisfy the cold content of the snowpack.
Another way to calculate the cold content is the procedure proposed by the Army Corps of Engineers in 1956. They define the cold content of snow (W sub c) as the heat required per unit area to raise the temperature of the snowpack to 0 degrees C, expressed as millimeters of liquid water, which upon freezing within the snowpack, will warm the snow to 0 degrees C through the release of the latent heat of fusion:

Old school hydrology!