 SNOW HYDROLOGY (GEOG 4321): SNOW FORMATION IN THE ATMOSPHERE
SNOW HYDROLOGY (GEOG 4321): SNOW FORMATION IN THE ATMOSPHERE
Instuctor: Mark Williams
Telephone: 492-4794 or 492-8830
Readings
- P. Hobbs, Ice in the Atmosphere
- Avalanche Handbook, pp 37-44
- Lind, Physics of Skiing, pp 17-20
- Handbook of Snow, Chapter 4.
History
Conditions for Precipitation
Nucleation
Ice Nuclei
Growth of Ice Particles
Artificial Snow
Types of Snow Crystals
Impurities in Snow
Storm Types
Questions
- Can there be identical snowflakes?
- Are snowflakes in mountains prettier than other places?
- Why is snow white?
- Why are holes in a snowpack blue?
(from "In Praise of Snow, by Cullen Murphy").
The symmetry of ice crystals was commented upon by the
Chinese
in the second
century B.C. Europeans had recorded the same observation at least by the
Middle Ages. The intellectual pedigree of snow scholarship in the West is
distinguished. The Dominican scholastic Albertus Magnus wrote about snow
crystals in the thirteenth century. At the beginning of the seventeenth
century the same subject beguiled Johannes Kepler. "There must be some
definite cause," he wrote in 1609, shortly after making the discovery that
the planets travel not in circles but in ellipses, "why, whenever snow begins
to fall, its initial formation invariably displays the shape of a
six-cornered starlet. For if it happens by chance, why do they not fall just
as well with five corners or with seven?" In his pamphlet Kepler drew
parallels with honeycombs and the pattern of seeds inside pomegranates, but
was unable to explain the flakes' hexagonal form. Somewhat later Rene
Descartes discerned that branches sprout off each side of the stems of
hexagonal snowflakes at an angle of 60 degrees, with an angle of 120 degrees
thus separating the branches themselves. The process is complex, but the
hexagonal shape of snowflakes essentially reflects the underlying atomic
structure of water. One suspects that even the skeptic Descartes would have
offered up a Te Deum had he known that the two hydrogen atoms in a molecule
of water branch off the oxygen atom with about 120 degrees of separation.
For all the scientific awareness of the symmetrical character of snow
crystals, the ubiquity of their popular image--the one we see in children's
paper cutouts and on bags of ice and signs for motels that have
air-conditioning--is a relatively recent phenomenon. What snowflakes actually
looked like was not widely known until the middle of the nineteenth century,
when the book Cloud Crystals, with sketches by "A Lady," was published in the
United States. The lady had caught snowflakes on a black surface and then
observed them with a magnifying glass. In 1885 Wilson Alwyn ("Snowflake")
Bentley, of Jericho,
Vermont, began taking photographs of snowflakes through
a microscope. Thousands of Bentley's photomicrographs were eventually
collected in his book Snow Crystals (1931). The fact that not one of the
snowflakes photographed by Bentley was identical to another is probably the
basis for the idea that no two snowflakes are ever exactly the same--an idea
that is in fact unverifiable.
(from Hornberger et al., 1998):
There are three primary steps in the generation of precipitable
water in the atmosphere: (1) creation of saturated conditions
in the atmosphere; (2) condensation of water vapor into liquid
water; and (3) growth of small droplets by collision and coalescence
until they become large enough to precipitate.
Saturated conditions typically
arise in the atmosphere when an air mass is cooled by
being lifted vertically. The vapor pressure e
is a measure of how much water vapor is in the air. The
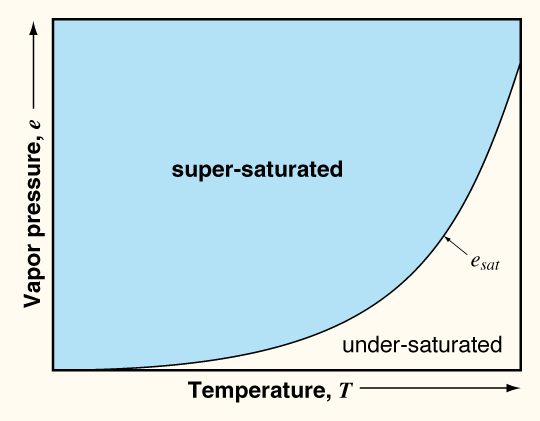 saturation vapor pressure is the value of e
for saturated conditions. This value is strongly dependent on
the air temperature; that is, warm air can hold more water vapor,
or, conversely, cool air cannot hold as much water vapor as warm
air. Thus, cooling an air mass tends to produce saturated
conditions, because esat is reduced. Cooling
can happen in a number of ways, such as when air masses rise over
mountains or other topographic features (referred to as orographic cooling),
or warm air masses rise
above cooler air masses at fronts.Heating of the Earth's surface
(especially during the summer) can make air near the surface less
dense so that it rises and cools, often producing thunderstorms.
saturation vapor pressure is the value of e
for saturated conditions. This value is strongly dependent on
the air temperature; that is, warm air can hold more water vapor,
or, conversely, cool air cannot hold as much water vapor as warm
air. Thus, cooling an air mass tends to produce saturated
conditions, because esat is reduced. Cooling
can happen in a number of ways, such as when air masses rise over
mountains or other topographic features (referred to as orographic cooling),
or warm air masses rise
above cooler air masses at fronts.Heating of the Earth's surface
(especially during the summer) can make air near the surface less
dense so that it rises and cools, often producing thunderstorms.
Condensation is simply the phase change whereby water vapor becomes
liquid water. It requires not only the creation of saturated
conditions within the air, but also the presence of condensation
nuclei, small particles such as dust or previously formed water
or ice particles. Condensation may produce such small particles
that they remain stable in the atmosphere. The white clouds observed
on a fair day, for example, are composed of water droplets that
are too small to precipitate. It is the coalescence of
small droplets into larger drops, through collision of
small droplets with each other or with larger drops, that gives
rise to precipitable raindrops that are large enough to overcome
gravity and fall to the ground as rain.
Snow differs from rain in a very important manner.
Particules in the atmosphere must meet very specific
requirements to act as ice nuclei.
The reason for these exacting requirements is that
it is very difficult to "make" water molecules
line up correctly to form a crystalline, 3-dimensional shape.
The limiting ingredient to form snow in the atmosphere
is often nucleating agents.
That's why cloud seeding works.
Thus, for snow to form in the atmosphere,
we need:
- Water present in the vapor phase
- RH => to 100%
- Tair <= to 0 degree C
- Nucleating agents.

What happens if we meet all the requirements except the presence
of nucleating agents?
Is the amount of water vapor capped at a RH of 100%?
The answer is no, the amount of water vapor in the atmosphere
can continue to increase above a RH of 100%.
When that occurs,
we say the atmosphere is supersatured,
that is, the amount of water vapor in the atmosphere exceeds 100%.
The amount of supersaturation is often expressed as the actual
relative humidty minus 100%.
Thus, if the actual RH equals 112%, the amount of supersaturation is 12%.
You'll often see diagrams of crystal shape and size with axes of air temperature
and vapor density rather than vapor pressure.
Remember, vapor density and vapor pressure are interchangeable through
the Ideal Gas Law.
Vapor density is the preferred unit because our real interest is the mass
of water that moves from the gas phase to become snow.
- Homogeneous Nucleation
- Spontaneous process.
- No nuclei necessary.
- formation of ice crystals from supercooled
water droplets occurs at -40 deg C (also -40 deg F).
- Heterogeneous Nucleation
- Deposition Nucleus
- Growth by deposition from vapor phase onto existing
ice nucleus.
- Radius > 1000 angstroms
- Immersion-Freezing Nucleation
- Ice nucleus imbedded within a super-cooled water droplet.
- Radius > 100 angstroms.
- Contact Nucleation
- Collosion contact between ice nucleus and
supercooled water droplets.
- More efficient than freezing nucleation.
- Same particle nucleates at temperature that are
5-10 deg C warming than in freezing nucleation.
- No good explanation as to why.
- Definition
- Deposition of gaseous or liquid water onto
an ice nuclei.
- Size
- 0.5 to 8.0 micrometers in diameter.
- Can be advected thousands of miles.
- dust storms, volcanic eruptions good sources.
Number of ice nuclei in the atmosphere,
on average, is:
ln N = A(T1 - T)
where T1 is the temperature for a concentration
of 1 active ice nucleus per liter and A varies from about
0.3 to 0.8.
Typically, T1 is about -20degC.
Since the total number of particles in a liter
of air is about 108,
only about 1 in 108 airborne particles
is effective as an ice nucleus at -20degC.
Consequently,
as a result of the low concentration of ice nuclei
in the atmosphere,
many clouds consist entirely,
or partially,
of supercooled water droplets.
If we take A=0.6,
the above equation predicts that the concentration
of ice nuclei increases by about an order
of magnitude for every 4degC fall in temperature.
The surface of the earth appears to be the
primary source of ice nuclei in the atmosphere.
- Silicate minerals an important source
- Clays such as kaolinite and illite
- In Greenland, 85% of ice crystals had clay particle
in their center, with
- more than half the clay particles identified as kaolinite.
- Sizes of ice nuclei ranged from 0.5 to 8 um in diameter.
- Sea salt is a poor nucleating agent.
Cloud drops must be cooled to at least -35degC before
sea salt aerosols will act as ice nuclei.
Organic Ice Nuclei (from LTER CED newsnotes)
- Decomposing leaf matter appears to make excellent ice nuclei.
- Microbial decomposition substantially increases the
effectiveness of the ice nuclei.
- Aerobic decomposition is much better than anaerobic
decomposition in making ice nuclei.
- Humus matter more than one year old nuclei that worked
at temperatures of -5 deg C.
- Newer organic matter had ice nuclei that worked at
temperatures of -15 to -20 deg C, much older and hence
much less efficient than more decomposed and older humus.
- Decaying leaf material makes about 1010 ice
nuclei per gram of decaying leaf material, about 0.1% of
the mass of the orginal material.
- Particle size ranged from 0.1 to 0.05 micrometers.
- Fluxes of ice nuclei from 10 to 1,000 ice nuclei per
square centimeter per day have been reported.
- Fluxes of ice nuclei from organic matter go to near
zero as when snow covers the ground.
- Composition of organic ice nuclei:
- Insoluble in water.
- Stable in all common organic solvents.
- Temperatures above 60 deg C deactivates the ice nuclei.
References
- Mason, BJ, The shapes of snow crystals---fitness
for a purpose?, QJRMS, V 120, pp 849-860, 1994.
- Schnell, R. C. and G. Vali, Atmospheric ice nuclei
from decomposing vegetation, Nature, V 236, pp 163-165, 1972.
- Schnell, R. C. and G. Vali, World-wide source of of
leaf-derived freezing nuclei, Nature, V 246, pp 212-213, 1973.
- Schnell, Bul of Am. Metero. Soc, V 55(6), pp 670, 1974.
- Schnell, R. C. and G. Vali,
Biogenic ice nuclei: part I. Terrestrial and Marine Sources,
J. Atm. Sci., V33, pp 1554-1564.
- Schnell, R. C. and G. Vali,
Biogenic ice nuclei: part II. Bacterial Sources,
J. Atm. Sci., V33, pp 1565-1570.
- Fall, R., and P.K. Wolber, Biochemistry of bacterial
ice nuclei, in Biologilcal Ice Nucleation and Its Applications,
ed by R.E. Lee, Jr., G. J. Warren, and L. V. Gusta, pp 63-83,
APS Press, St Paul, MN, 1995.
- Chen, J. and V. Kevorkian, Heat and Mass Transfer
in Making Artificial Snow, Ind. Eng. Chem. Process Des. Develop.
V 10, p 75, 1971.
Growth from the vapor phase
- Driving Mechanism
- Supersaturation is always greater over ice than
over water at the same temperature.
Supersaturation in cloudy air with respect
to liquid water is generally less than 1%.
However, this corresponds to supersaturation
with respect to ice of about 10% at -10degC and
21% at -20degC.
- Net Result
- Flux of water vapor liquid phase to ice phase
- Flux of heat from ice to liquid phase produced
by latent heat of fusion.
- Growth Rate
- Maximum at -12 to -15 deg C.
- Grows rapidly for about 1/2 hour, then
relatively slowly.
- Maximum Size
- Maximum mass is tens of micrograms.
- Maximum diameter is tenths of millimeters.
- Cannot produce large snowflake.
- Produces only drizzle-size raindrops.
- Large snowflakes must be grown by aggregration and riming.
Aggregation
- Definition
- Snowflakes formed by the collosion and adhesion
or sticking of ice and snow crystals.
- Growth Rate
- Fast compared to vapor diffusion.
- Can grow from 1 millimeter to 10 millimeters
in about 20 minutes in a cloud with high ice content.
- Maximum Size.
- At -1 to -4 degC there is a substantial psuedo-liquid
film on the ice surface.
This promotes formation of an ice-neck connection
between crystals.
Crystals freeze together and are mechanically locked.
- Mass is sufficient for snowflakes to fall from
the atmosphere to the ground, or to undergo sedimentation.
Riming
- Definition
- Adhesion of a super-cooled water droplet to an
ice particle or snow crystal.
- Size of Supercooled Water Droplets
- 2-50 micrometers in diameter.
- Adhesion Efficiency
- Very high, approximately one, eg every super-cooled
water droplet adheres or sticks to every ice particle
or snow crystal it comes in contact with.
- Growth Rate
- Relatively fast.
A single snow or ice crystal can grow from a
250 micrometer radius to 1-2 millimeter radius
in 10-20 minutes.
- Formula for Calculatin Growth Rate.
- dm/dt = pie r2 a b w W
- r = radius of crystal;
- a = adhesion efficiency, usually 1;
- b = collosion rate between water droplets and ice crystals;
- w = crystal fall velocity relative to water droplets;
- W = liquid water content of cloud.
Types of Riming
- Rimed
- Initial form of crystal is apparent.
- Graupel
- Original crystal shape obliterated,
usually a round ball.
Also called soft hail, snow pellet.
- Hail
- More advanced riming.
- Sleet
- sleet: partially melted and refrozen snow crystals or
hard and transparent ice particles of frozen drops.
Kelly Doyle put together a nice powerpoint on snowmaking.
Please download and read
this powerpoint presentation on making snow
Problem
- Water droplets have a "hang-time of about 15 seconds".
- Air temperatures relatively warm, close to 0 deg C.
- Natural air humidity low, less than 100% RH.
Process
- Inject mixture of air and water from "snow-gun"
into the atmosphere.
- Produces liquid water droplets 100-700 microns in diameter.
- Cooling of air mass by adiabatic expansion.
The temperature of air and water shot out of the snowgun
will be lower than the ambient temperature by several degrees.
- Add nucleating agents that are efficient at warm
temperatures near 0 deg C.
- Makes good ski base.
- Produces rounded ski grains that pack efficiently.
- High density snow, 400-450 kg/m^3.
Bacterial Ice Nuclei
- Pseduomonas syringae is commercial bacteria.
- Bacteria.
- Isolated from corn plant.
- Cultivated and then freeze-dried, preserving
cellular structure intact.
- Protein in cell wall the active nucleating agent.
- Hexagonal platelets formed from proteins in cell wall.
- Bond length between or nitrogen atoms comparable to
bond length between oxygen atoms in ice.
- Hydrogen bonds hold tertiary shape of proteins.
- Exact mechanism of nucleating process unknown.
- P. syringae very efficient nucleating agent
- 2.5 x 10^5 nucleation sites per mL of water from
with 0 to 100 naturally occurring nucleations.
- High fidelity of protein chains helps.
- Helical structure of protein also helps.
- Raises freezing temperature of super-cooled water
droplets about 6 deg C.
References
- Dave Lind's book, pp 17-20.
Factors that determine shape of snow crystals.
- Temperature
- Percent supersaturation
- Ice nuclei type
Growth directions
- Basal Plane
- three a axes;
- each 120 deg apart;
- hexagonal symmetry;
- produces plate-like and star-like structures.
- Optic Axis
- c axis;
- 90 deg to basal plane;
- produces needle or columnlike structures.
- High supersaturation
- Growth occurs where excess vapor density is highest.
- Growth occurs at edges and corners.
- Complicated crystals such as dendrites.
- Low supersaturation
- Produces solid structures.
- Atmospheric history after deposition.
- Ice crystals and snowflakes often pass through
different temperature and water vapor regimes
as they pass through the atmosphere.
Crystal type can change after initial formation.
- Capped columns are an example.
Solid column is formed in cold air with low
supersaturation.
As the solid column enters warmer air, plates
may grow on end to make a "capped column".
Overview of crystal types and growth patterns.
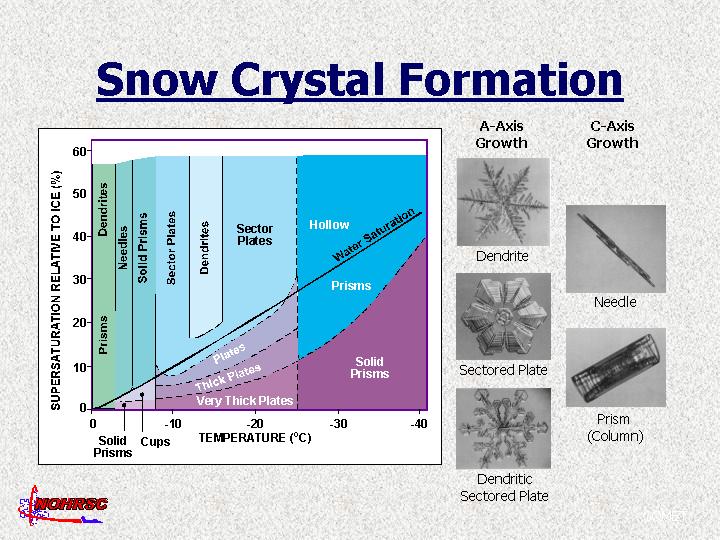
Examples of snow crystal types.
Incorporation in the ice lattice.
- Ice is a poor solvent (in constrast to liquid water).
- Impurities which are dissolved in water which freezes
in the atmosphere generally:
- are rejected, or;
- precipitate.
- Incorporation of impurities into snow involves:
- vacancy substitution, the replacement of a water
molecule with one that has a similar ionic radius
and charge, such as ammonia (NH3 for
H2O, or florine (F) for oxygen (O).
- interstitial fit, accomodation in the open lattice
space among water molecules, such as KOH.
- Certain ions are more easily incorporated into solid water:
F, NH3, K
- Certain ions are not easily incorporated and rarely found
inside the crystalline lattice, including sulfate and nitrate.
Incorporation on outside of snow and ice crystals.
- Snow and ice crystals have a very high surface to volume
ratio relative to rain.
- Snow and ice crystals generally have a much lower settling
rate than rain.
- Hence snow and ice crystals are much better at scavenging
impurities out of the atmosphere than rain.
- Snow and ice crystals thus have many of the impurities
located on the outside of the particle rather than incorporated
into the interior of the crystalline lattice structure.
Storm Types

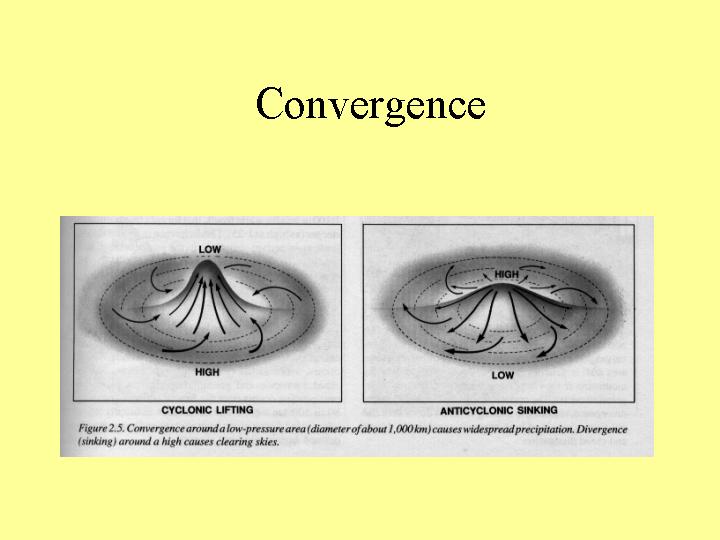

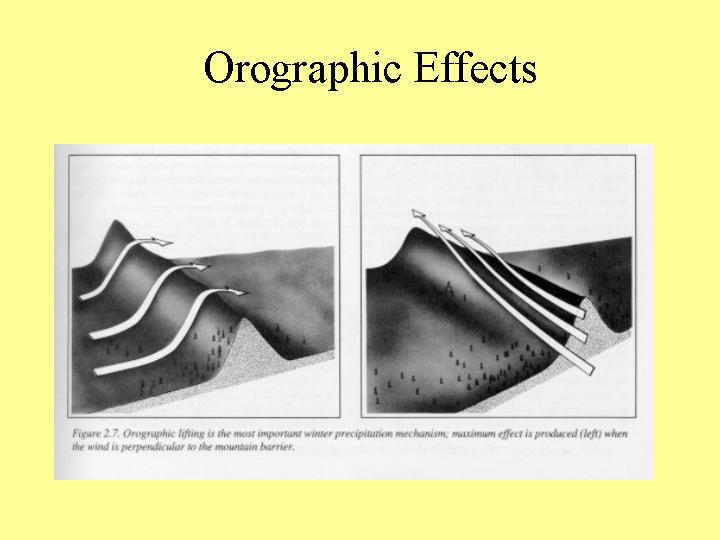

References
Here's a nice simple description of snow formation
in the atmosphere, from the Teel family:
Simple description of snow formation in the atmosphere
Here's another nice simple description of snow formation
in the atmosphere, this one from USA Today:
Simple description of snow formation in the atmosphere
Here's a slightly more detailed description of snow formation
in the atmosphere, this one from NCAR/UCAR:
Snow formation in the atmosphere: Science Now article
from UCAR/NCAR
Another nice description of snow formation in the atmosphere:
Jerry Dennis on Snowflakes and Crystals
 SNOW HYDROLOGY (GEOG 4321): SNOW FORMATION IN THE ATMOSPHERE
SNOW HYDROLOGY (GEOG 4321): SNOW FORMATION IN THE ATMOSPHERE  SNOW HYDROLOGY (GEOG 4321): SNOW FORMATION IN THE ATMOSPHERE
SNOW HYDROLOGY (GEOG 4321): SNOW FORMATION IN THE ATMOSPHERE  saturation vapor pressure is the value of e
for saturated conditions. This value is strongly dependent on
the air temperature; that is, warm air can hold more water vapor,
or, conversely, cool air cannot hold as much water vapor as warm
air. Thus, cooling an air mass tends to produce saturated
conditions, because esat is reduced. Cooling
can happen in a number of ways, such as when air masses rise over
mountains or other topographic features (referred to as orographic cooling),
or warm air masses rise
above cooler air masses at fronts.Heating of the Earth's surface
(especially during the summer) can make air near the surface less
dense so that it rises and cools, often producing thunderstorms.
saturation vapor pressure is the value of e
for saturated conditions. This value is strongly dependent on
the air temperature; that is, warm air can hold more water vapor,
or, conversely, cool air cannot hold as much water vapor as warm
air. Thus, cooling an air mass tends to produce saturated
conditions, because esat is reduced. Cooling
can happen in a number of ways, such as when air masses rise over
mountains or other topographic features (referred to as orographic cooling),
or warm air masses rise
above cooler air masses at fronts.Heating of the Earth's surface
(especially during the summer) can make air near the surface less
dense so that it rises and cools, often producing thunderstorms.




